Of The Molecules Pf3 And Pcl3, Which Has Bonds That Are More Polar?
Who Has The Highest Psr Rating Ever 2022, 2K MOBILE IS 10x BETTER THAN NBA 2K22! (NEW PARKS), 11.63 MB, 08:28, 130,534, COLETHEMAN, 2021-12-17T00:57:12.000000Z, 19, Yamaha PSR-E373 keyboard review – TrendRadars, www.trendradars.com, 590 x 410, png, , 5, who-has-the-highest-psr-rating-ever-2022, KAMPION
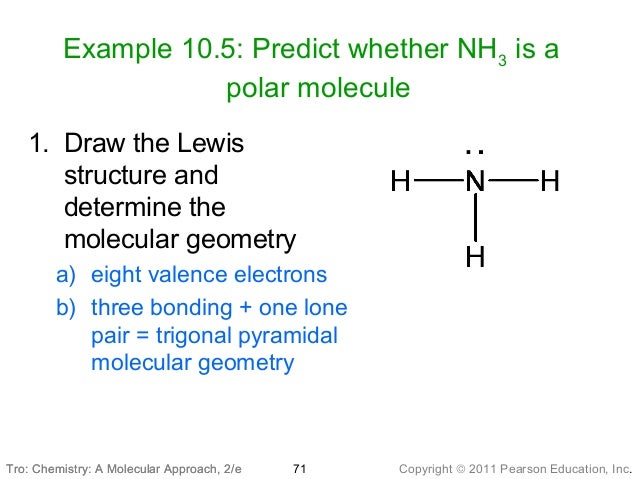
In the molecule hcl, which atom is the negative pole? H part b of the molecules pf3 and pcl3, which has bonds that are more polar? In which direction should the polarity arrows point? To the right b.
According to vsepr theory, the pf3 molecule created a geometrical shape of a trigonal pyramid. An asymmetrical angle of roughly 96. 3° will result from the two polar atom bond pairs. A nonpolar molecule has a symmetric structure, whereas pf3 has an asymmetric shape, which makes it a polar molecule. Of the molecules pf3 and pcl3, which has bonds that are more polar? Of the molecules pf3 and pcl3, which has bonds that are more polar? Get 20% off grade+ yearly subscription → The dipole moment of a polar molecule is always equaled to non zero and nonpolar molecules always have zero dipole moment. Pcl3 is a polar molecule therefore its dipole moment is 0. 97 d. The geometrical shape of the molecule is an important and physical parameter that helps to determine the polarity of a molecule.
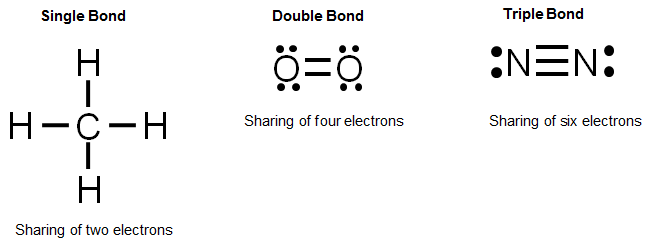
Lewis Dot Diagram For Pcl3 - Wiring Diagram

Lewis Dot Diagram For Pcl3 - Wiring Diagram
Download Nh3 Molecular Geometry Polarity Images - GrAffiTi
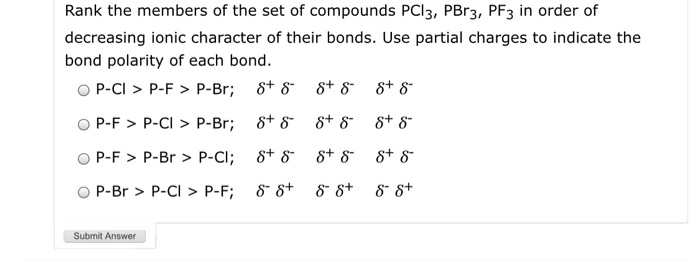
Solved: Rank The Members Of The Set Of Compounds PCl3, PBr... | Chegg.com
Is NF3 polar or nonpolar? - Quora
Lewis Dot Diagram For Pcl3 - Wiring Diagram
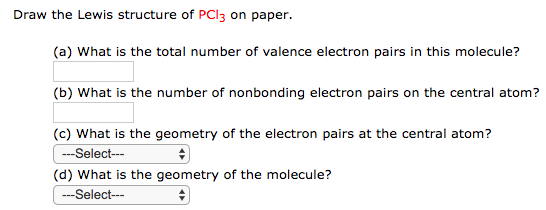
38+ Geometry And Polarity Of Some Molecules Images - GrAffiTi
117 Practice Problems – Chapter 13

The Shapes Of Molecules

Komentar
Posting Komentar